Course Title CHE INORGANIC. Part G Which chemicals generate the following colors in the fireworks The.
How Do Fireworks Get Their Colors Human World Earthsky
You may use chemical formulas rather than common names of compounds in your table.

. And sodium yields yellow. Chlorine Chlorine is an important component of many oxidizers in fireworks. You need to use chemical formulas rather than common names of compounds in your table.
Strontium and sodium produce brilliant orange. Mineral elements provide the color in fireworks. Strontium yields deep reds.
Which chemicals generate the following colors in the fireworks Type your from SCIENCE 101 at Grace King High School. Write the chemical formula for the following compounds used to create these features. Additional colors can be made by mixing elements.
Calcium salts produce orange fireworks. Other colors can be made by mixing elements. Diantimony trisulfide glitter effect b.
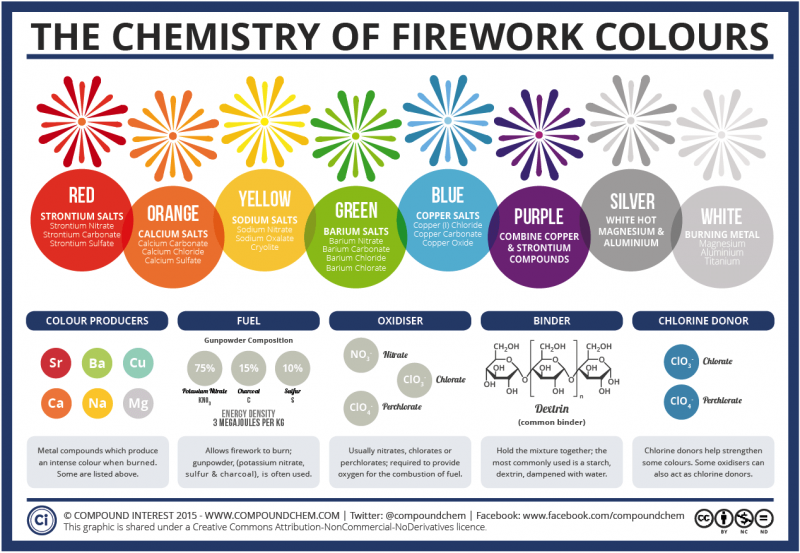
Color Produced Elements Primary mineral ores bright greens barium barite deep reds strontium celestite blues copper chalcopyrite yellows sodium halite rock salt brilliant orange strontium sodium celestite halite silvery white titanium zirconium magnesium. Strontium and Copper Orange. The chemicals responsible for the red flame have been strontium the green flame has been produced by barium and sodium has been responsible for the yellow flame.
Carbon provides the fuel for a firework. William nitrate and orange yellow color very included a green color. Aerial fireworks are produced by chemical changeThe fireworks have been composed of gun powder and chemicals which provide color to the reactionThe gun powder has been composed of.
Wariber 46 5 months ago. Copper and strontium make lavender. Copper salts and chlorine Green.
Create a table that lists the metals often used as salts that are used as coloring agents to create the following colors of fireworks. Aluminum Aluminum is used to produce silver and white flames and sparks. Carbon Carbon is one of the main components of black powder which is used as a propellant in fireworks.
Calcium salts produce orange fireworks. Zinc oxide smoke c. Part g which chemicals generate the following colors.
Strontium salts lithium salts lithium carbonate Blue. Create a table that lists the chemical compounds that create the following colors of fireworks. What are used by Fireworks to create different colour - 19026732 1.
You may use chemical formulas rather than common names of compounds in. Type your response here. Copper and strontium compoundsred.
Calcium is used to deepen firework colors. Mixture of strontium red and copper blue Orange color of fireworks is due to calcium and not due to carbon. Create a table that lists the chemical compounds that create the following colors of fireworks.
Type your response here. Include the element name and symbol. Rest colors and respective metals are matched correctly.
Strontium burns red copper makes blue barium glows green sodium yellow calcium salt orange incandesence of iron with carbon chacoal or. Sodium Salts Sodium Chloride Purple. Mixture of strontium red and copper blue Orange.
Common forms include carbon black sugar or starch. School Camden High Camden. Strontium or lithium Blue.
Metal salts commonly used in firework displays include. Strontium and Lithium Blue. Blue turquoise yellow pink red brilliant red green bright green purple white.
The colors associated with fireworks are due to chemicals that are added to the fireworks that air non explosive chemicals that change that UM it colors when they are in the presence of the high energy of the burning fireworks such as strong team carbonate producing a red color calcium chloride and orange colors. Blue turquoise yellow pink red brilliant red green bright green purple white. Beastboyshubshivam beastboyshubshivam 02072020 Chemistry Secondary School 5 pts.
Calcium Calcium is used to deepen firework colors. Ammonium chloride white smoke d. Type your response here.
How Fireworks Work Anatomy of a Firework Chemistry of Fireworks Chemistry of. Carbon provides the fuel for a firework. List of colors and elements in Fireworks.
Which chemicals generate the following colors in the fireworks. Titanium Aluminum Magnesium. Pages 6 This preview shows page 5 -.
Blue turquoise yellow pink red brilliant red green bright green purple white. The colors are produced by heating metal salts such as calcium chloride or sodium nitrate that emit characteristic colors. Which chemicals generate the following colors in the fireworks.
Carbon is one of the main components of black powder which is used as a propellent in fireworks. Mineral elements provide the color in fireworks. Aluminum titanium or magnesium.
Blue yellow orange red green purple white and silver or electric white. Strontium carbonate red fireworks calcium chloride orange fireworks sodium. Create a table that lists the chemical compounds that create the following colors of fireworks.
Barium produces bright greens. Which chemicals generate the following colors in the fireworks. Titanium zirconium and magnesium alloys make silvery white.
In addition to vivid colors fireworks also use other visual features such as smoke glitter effects or delays in the firing train. After completing your research use the table that you created to design your.

Chemistry Of Fireworks Chemistry Of Fireworks Chemistry Science Chemistry

How Do Fireworks Get Their Colors Human World Earthsky

What Minerals Produce The Colors In Fireworks Fireworks Firework Colors How To Make Fireworks
0 Comments